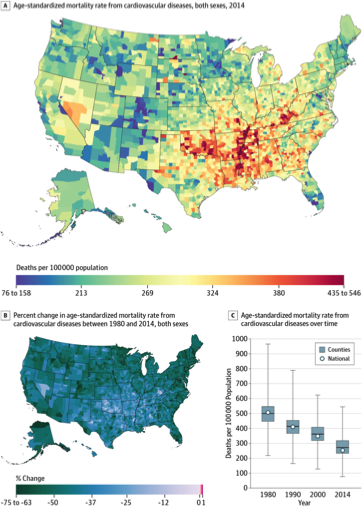
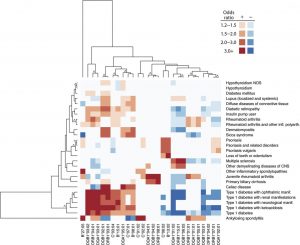
New Text – a Valuable Resource
The recently published text, Drug Utilization Research: Methods and Applications, represents a formidable accomplishment, pulling together a comprehensive range of information on all topics related to this key area. With 48 chapters, it addresses study design, data collection, secondary data sources, classification systems, measurement units, drug expenditures, statistical methods, data visualization, and much more. I was happy to see a chapter addressing issues around pediatric drug utilization, and chapters on drug utilization of pregnant women and older populations, as well.
From the publisher’s website:
Drug Utilization Research (DUR) is an eclectic scientific discipline, integrating descriptive and analytical methods for the quantification, understanding and evaluation of the processes of prescribing, dispensing and consumption of medicines and for the testing of interventions to enhance the quality of these processes. The discipline is closely related and linked mainly to the broader field of pharmacoepidemiology, but also to health outcomes research, pharmacovigilance and health economics.
Drug Utilization Research is a unique, practical guide to the assessment and evaluation of prescribing practices and to interventions to improve the use of medicines in populations. Edited by an international expert team from the International Society for Pharmacoepidemiology (ISPE), DUR is the only title to cover both the methodology and applications of drug utilization research and covers areas such as health policy, specific populations, therapeutics and adherence.
The only way to assemble such a book is with a strong group of editors, Monique Elseviers, Björn Wettermark, Anna Birna Almarsdóttir, Morten Andersen, Ria Benko, Marion Bennie, Irene Eriksson, Brian Godman, Janet Krska, Elisabetta Poluzzi, Katja Taxis, Vera Vlahovi?-Pal?evski, Robert Vander Stichele are all accomplished researchers in this field.