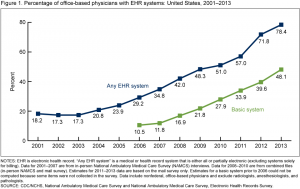
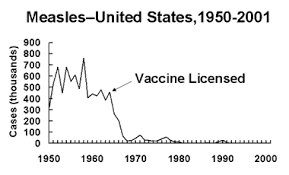
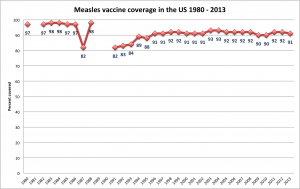
For the third year, the ISPE annual meeting (ICPE 2017 Montreal) will offer the pre-conference course in Pediatric Pharmacoepidemiology.
Mark your calendars for Sunday, August 27, 2017 (register here).
Brief Overview of Course
The increasing use of medications by children and the history of excluding children from clinical trials have created the need for pediatric pharmacoepidemiology, a sub-specialty within pharmacoepidemiology. Unique challenges in studying children, accessing data, defining outcomes, and designing studies require specialized methodologic skills and operational approaches. This half-day course will introduce participants who have a good understanding of pharmacoepidemiology to the specialized methodologic and operational approaches used to study medications in children.
Age is not a simple variable when studying children
Educational Objectives
The course will be taught in an interactive format with ample opportunities for questions and discussion. Regulatory issues around use of medications in children will be highlighted throughout the course. Participants will gain an understanding of key issues in pediatric pharmacoepidemiology including:
- An overview of pediatric pharmacoepidemiology and how it differs from pharmacoepidemiology in older age groups
- Study design and databases to monitor safety
- Defining, measuring and validating outcomes in pediatric pharmacoepidemiology
- Variables critical to pediatric pharmacoepidemiology such as month of birth, seasonality, growth and development
Target Audience
ICPE attendees interested in gaining a basic understanding of the need for and approaches to pediatric pharmacoepidemiology.
Course Faculty/Presentations
- Tamar Lasky, PhD Owner, MIE Resources Why Pediatric Pharmacoepidemiology? Age Sub-groups in Children: Methodologic Considerations
- Alan Kinlaw, PhD Post-doctoral Research Fellow, Sheps Center for Health Services Research, University of North Carolina at Chapel Hill Leveraging granular birthdate information for studies of young children
- Rachel E. Sobel, DrPH Senior Director, Epidemiology, Worldwide Research and Development / Pfizer Inc. Monitoring Drug Safety: Study Design, Data Sources and Case Study
- Timothy Beukelman, MD, MSCE Associate Professor of Pediatrics, Division of Pediatric Rheumatology, Department of Pediatrics, University of Alabama at Birmingham The Need for Validation in Pediatric Outcomes
- Daniel B. Horton, MD, MSCE Assistant Professor of Pediatrics and Epidemiology, Rutgers University Growth and Development: Variables of Particular Importance in Pediatric Pharmacoepidemiology